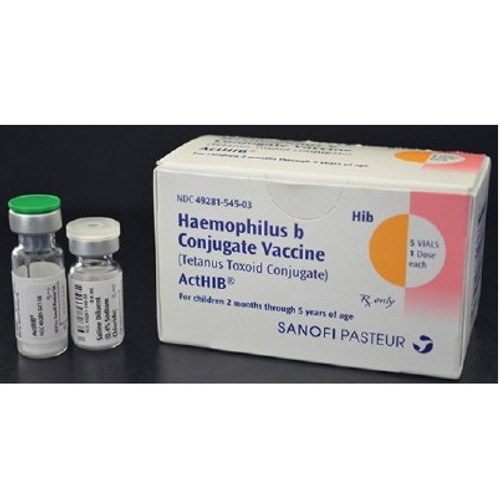
- Indicated for the prevention of invasive disease caused by Haemophilus influenzae type b
- Approved for use as a four dose series in infants and children 2 months through 5 years of age
- Single Dose Vial
- Injection
- NDC Number: 49281-0545-03
- Requires Refrigeration
- 10 mcg / 0.5 mL Strength
- Intramuscular
- For use in Children 2 Months to 5 Years of Age